Applied Spectroscopy
An International Journal of Spectroscopy
A journal of the Society for Applied Spectroscopy—over 70 years of scientific excellence and education
To gain full access to Applied Spectroscopy, please log in with your SAS account.
Please use the following link to view abstracts and table of contents for the journal:
Complete List of Issues and Abstracts (1951 to present)
Applied Spectroscopy is one of the world's leading spectroscopy journals, publishing high-quality articles, both fundamental and applied, covering all aspects of spectroscopy. Established in 1948, the journal is owned by the Society for Applied Spectroscopy and is published monthly. The journal is dedicated to fulfilling the mission of the Society to “…advance and disseminate knowledge and information concerning the art and science of spectroscopy and other allied sciences.” All manuscripts are rigorously peer-reviewed.
The journal publishes high-impact reviews, original research papers, and technical notes. In keeping with the Society's educational mandate, Focal Point Review papers are free to view. This means that the articles are freely available at the time of publication to scientists, students, and the general public worldwide.
With an Impact Factor (IF) of 3.588, Applied Spectroscopy is in the top quartile of journals in the Instruments and Instrumentation category and in the top half of the Spectroscopy category.
Click here for further information on Applied Spectroscopy's Aims and Scope and manuscript submission guidelines.
Journal Highlights
Focal Point Reviews
As noted above, the journal publishes high-impact reviews, original research papers, and technical notes. In keeping with the Society's educational mandate, Focal Point Review papers are free to view.
May 2022 Focal Point Review
Chemometrics for Raman Spectroscopy Harmonization
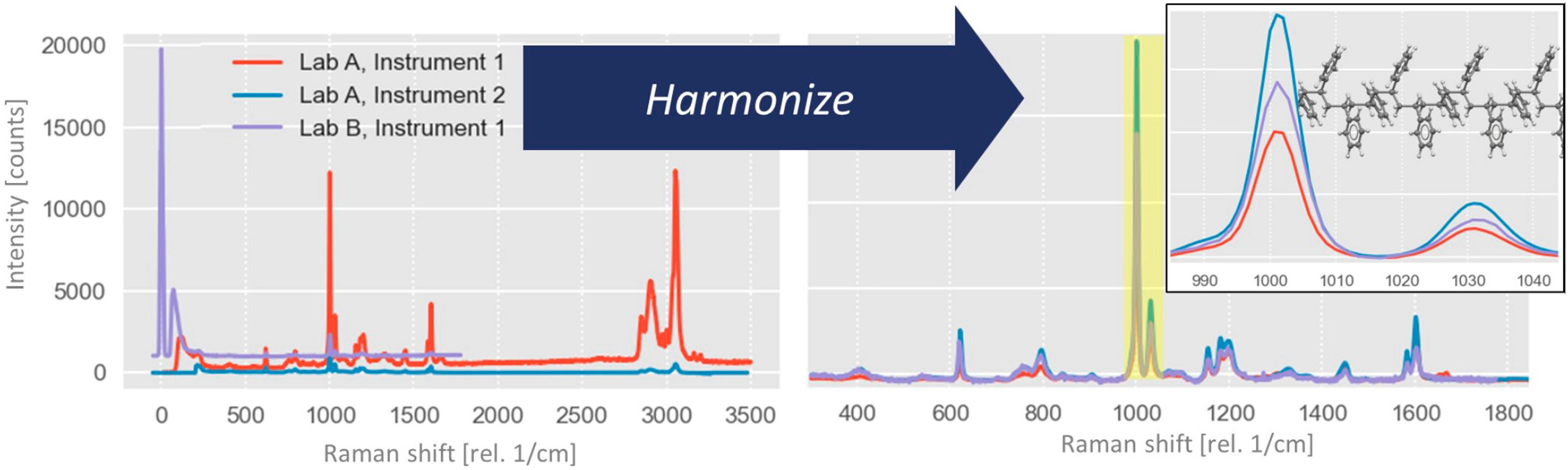
Raman spectroscopy is used in a wide variety of fields, and in a plethora of different configurations. Raman spectra of simple analytes can often be analyzed using univariate approaches and interpreted in a straightforward manner. For more complex spetral data such as time series or line profiles (1D), Raman maps (2D), or even volumes (3D), multivariate data analysis (MVDA) becomes a requirement. Even though there are some existing standards for creation, implementation, and validation of methods and models employed in industry and academics, further research and development in the field must contribute to their improvement. This review will cover, in broad terms, existing techniques as well as new developments for MVDA for Raman spectroscopic data, and in particular the use associated with instrumentation and data calibration. Chemometric models are often generated via fusion of analytical data from different sources, which enhances model discrimination and prediction abilities as compared to models derived from a single data source. For Raman spectroscopy, raw or unprocessed data is rarely ever used. Instead, spectra are usually corrected and manipulated,1 often by case-specific rather than universal methods.
Calibration models can be used to characterize qualitatively and/or quantitatively samples measured with the same instrumentation that was used to create the model. However, regular validation is required to ensure that aging or incorrect maintenance of the instrument does not alter the model’s predictions, particularly when applied in regulated fields such as pharmaceuticals. Furthermore, a model transfer may be required for different reasons, such as replacement or significant repair of the instrumentation. Modeling can also be used to consistently harmonize Raman spectroscopic data across several instrumental designs, accounting for variations in the resulting spectrum induced by different components. Data for Raman harmonization models should be processed in a protocolled manner, and the original data accessible to allow for model reconstruction or transfer when new data is added. Important processing steps will be the calibration of the spectral axes and instrument dependent effects, such as spectral resolution. In addition, data fusion and model transfer are essential for allowing new instrumentation to build on existing models to harmonize their own data. Ideally, an open access database would be created and maintained, for the purpose of allowing for continued harmonization of new Raman instruments using an outlined and accepted protocol.
Follow Applied Spectroscopy on Social Media!
If you have news you would like to share, please email the SAS Publicity Committee: sas.publicnews@gmail.com. The official journal hashtag is #AppliedSpec


.png)
